Our experience ranges from Clean Industries to Heavy Industrial Projects, from cGMP to non-cGMP facilities. QEDs’ design, documentation and site assistance capability covers Pharmaceuticals, Medical Devices, Food Production, Cosmetics, Consumer Disposables, Heavy Industry, Vehicle Production, Engine Assembly, Warehouses, Logistics Centres and Coldrooms. Our expertise extends to ancillary facilities including Canteens, Training Centres, Administration Offices, Energy Centres, Workshops and Quality Control Centres.
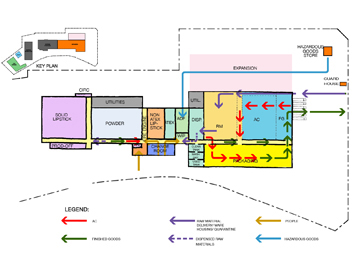
The scale of large industrial facilities presents unusual design and regulatory issues. Reconciling internal functional needs with fire compartmentation and fire engine access is paramount. Planning for very efficient flow of people and materials is essential. These schemes often require separation of unqualified and qualified raw materials and finished goods, work in process buffer areas, equipment wash and drying areas and separate clean equipment stores. We have designed for LEAN flow and use of RFID tag tracking systems.

Large Wheel Loader Production & Testing Facility
Tongzhou, Completed 2012

Logistics Centre Expansion >20,000 sqm
Shanghai, Completed 2012
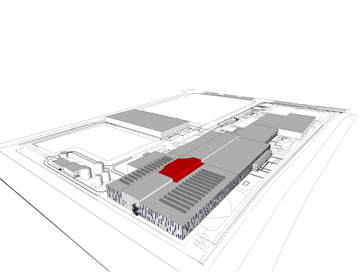
Cosmetics Production Facility Expansion Proposal
Suzhou, China 2011

Cosmetics Production Facility Expansion Proposal
Yichang, 2011


Cosmetics Production Facility Redevelopment Proposal
Shanghai, 2009

Greenfield Site, Wuxi 2016